Does Ice or Water Have More Potential Energy
When we place an ice cube in a glass of water the slow moving molecules in the. The potential energy is extracted from the steam and the steam molecules combine to form liquid.

River Formation Diagram Google Search Gravitational Potential Energy Geothermal Energy Energy Facts
Im confused because when I use the formula U frac32nRT they should all have the same U.

. As the water vapor gives up heat by conduction in the air the. The water has more thermal energy because the molecules of the water are moving faster than the molecules of the ice. The internal energy apparently increases.
The energy is the same but it changes from kinetic to potential energy. Similar explanation can be given to the Water-Steam transition. So at same temperature water molecule has more energy.
The water warms the ice because there is more thermal energy than ice and convection occurs where the neighboring particles collide with other neighboring particles. However if the question was which has the greater total energy the answer would be the gas as water vaper has higher potential energy than water liquid. Considering that shouldnt the potential energy DECREASE.
The average kinetic energy associated with liquid molecules is the same as the average kinetic energy of solid water molecules. Equivalently the free energy U-TS where U is the water energy S is the water entropy and T is the absolute. Since the temperature doesnt change all the thermal energy is used to increase the potential energyis this correct.
The thing that is confusing me is that when the latent heat of fusion is applied to the ice it breaks the. The ice has more thermal energy because it is a solid and the water is a liquid. Ice melts at 0 C to give water at 0 C.
This article is aimed to distinguish between these two types of ice. Now I know that water is MORE dense than ice. Strong winds that occur during a thunderstorm are the result of temperature differences between neighboring air masses.
The big energy change when water freezes is in the potential energy of interactions between the water molecules. Conversely you would need to lower the temperature of water considerably to form ice. Work can be extracted from the steam as it phase changes back to water without having a change in the temperature.
So this means there is much more energy in a kg of 100C steam than there is in a kg of 100C of water. Potential energy is the latent energy that could be released by the water and this increases because the water will release heat energy if it is frozen solid again. The boiled water now in a gaseous state is really hot and can be considered full of potential energy.
Δ U γ Δ A. Thermal energy is transferred to ice causing this to occur. The entropy of the water molecules goes down because their positions and orientations are constrained by the crystalline structure.
This would require a loss of heat and thus. When ice or any other solid melts its potential energy increases. In the liquid phase water molecules still have a weak bond and in the gaseous phase more modes of kinetic.
Temperature is defined as the measure of the average kinetic energy of a system. When the water temperature reaches around 0C the molecules s. Because water is open and ice is closed so water has more room for more energy particles.
The molecules of water have more energy than the molecules of ice at the same temperature this is because as ice is melted to the liquid state ice absorbed energy which is used in breaking the inter particles forces between the ice and hence water molecule have more kinetic energy. Why does liquid water have more energy than ice. Sorry but you would be wrong.
Molecules are constantly moving because they have energy. Less negative energy than a molecule in the center because it takes less work to free it from the droplet. As the liquid cools down the amount of potential energy is reduced and the molecules start to move slower.
We call the ratio Δ U Δ A γ the surface tension. I also know that average kinetic energy is directly linked to temperature so if they are at the same temperature the average kinetic energy should be the same. I said that it is greater than because when ice melts energy is added to break the potential energy bonds.
As the liquid cools down the amount of potential energy is reduced and the molecules start to move slower. A molecule near the surface has a higher ie. In a liquid form water molecules have more energy than in a solid they move around quickly essentially bouncing off of one another.
Freezing is a change in the ordering or structure of the. For example water is warmer than ice which means that the molecules in water generally have more kinetic energy and are thus moving faster than the ones in ice. Firstly why would the water molecules have potential energy by just staying at the surface.
It seems as though it has something to do with the gas having more degrees of freedom and thus a higher entropy. Now apparently the vapour has the highest internal energy the water has the next highest and the ice has the lowest internal energy. In the liquid the arrangement is less regular and the energy is not lowered as much.
So long as T. Indeed this is the only increase in energy since the thermal kinetic energy or temperature does not increase while melting. Therefore gas and vapor at the same temperature have the same kinetic energy.
Land ice and sea ice comprise the majority of the polar regions on Earth. When the water temperature reaches around 0C the molecules stick together and form a solid ice. That is how water and ice can be at the same temperature.
The most basic difference is that sea ice forms from salty ocean water whereas land ice ice sheets and glaciers form from fresh water or snowLand ice can be labelled as ice sheets whereas sea ice can be labelled as ice shelves. This is in fact potential energy. The water has more thermal energy because the ice has no thermal energy but the water does.
In the ice the molecules arrange to touch in a way that lowers this energy. How Does Water Turn Into IceWhy does water freeze and become ice.

Tj Potential Energy Is The Stored Or Pent Up Energy Of An Object Potential Energy Is Often Associated With Restoring Forces Such As A Spring Or The Force Of G

Gravitational Potential Energy An Overview Sciencedirect Topics

Phase Changes Boundless Physics

Kinetic Energy And Potential Energy Lesson Plans Potential Energy Energy Transfer Lesson Kinetic And Potential Energy

Http Thescienceteacher Co Uk Internal Energy Physics Lessons Science Teaching Resources Internal Energy

Entropy Vector Illustration Explanation Diagram Teaching Chemistry Physics Lessons Chemistry Classroom

Kinetic Vs Potential Energy Guided Practice Pdf Digital Versions Science Teaching Resources Potential Energy Physical Science Lessons

Energy Task Cards Scoot Potential Kinetic Transformations Gravitational Potential Energy Task Cards Energy Transformations
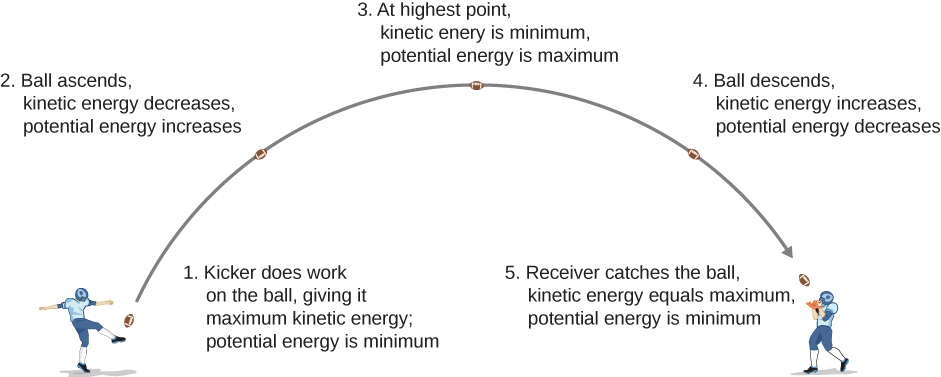
8 1 Potential Energy Of A System University Physics Volume 1

Using Potential Energy Diagrams Flv Physical Chemistry Potential Energy Fun Science

Thermodynamics Does The Potential Energy Increase When Temperature Is Raised Chemistry Stack Exchange

Specific Heat Of Water Very High So It Takes More Energy To Increase The Temperature Of A Given Mass Of Wat Teaching Science Science Facts Homeschool Science

Thermal Energy Science Information Text Thermal Energy Reading Comprehension Practice Reading Passages
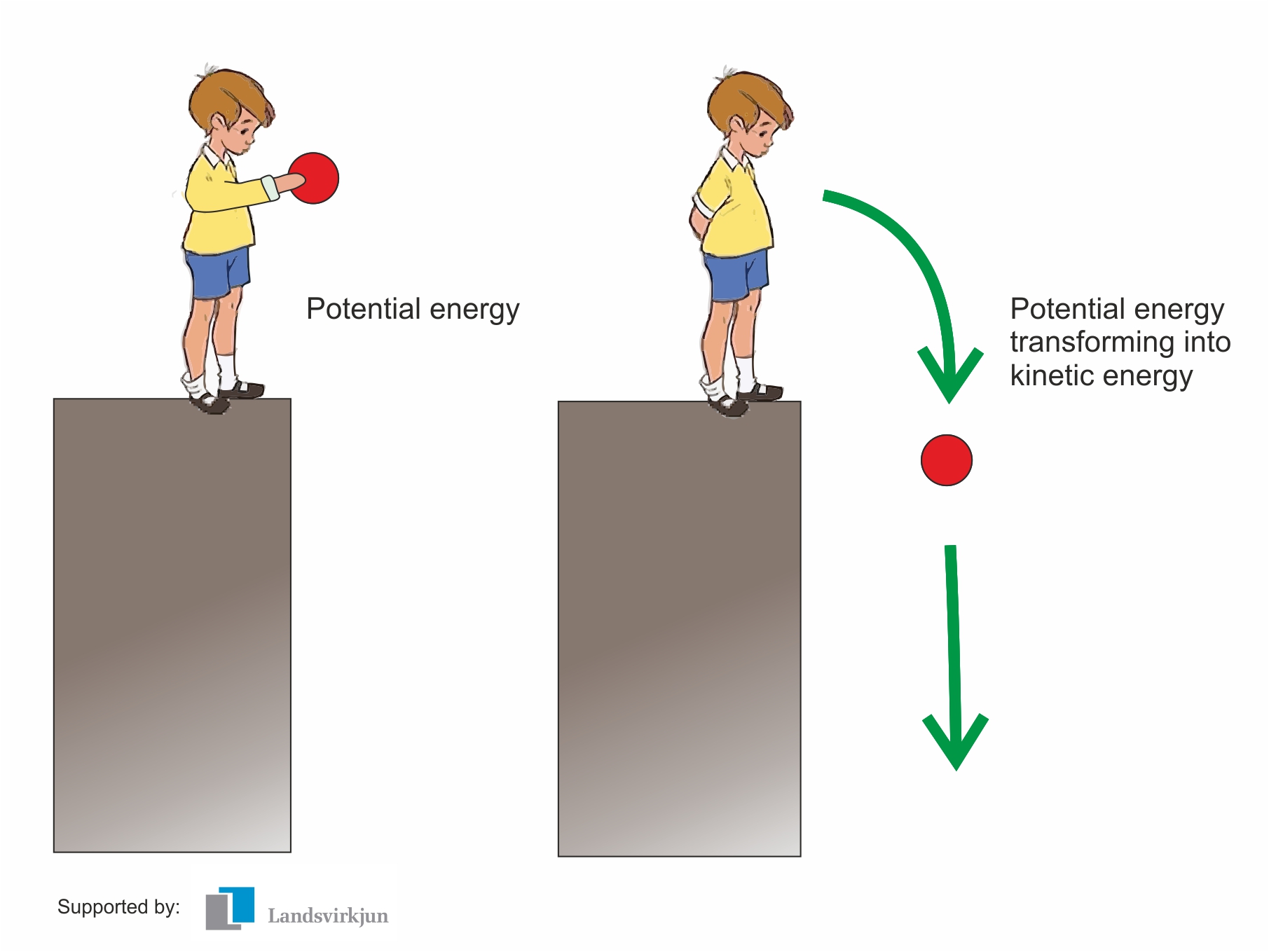
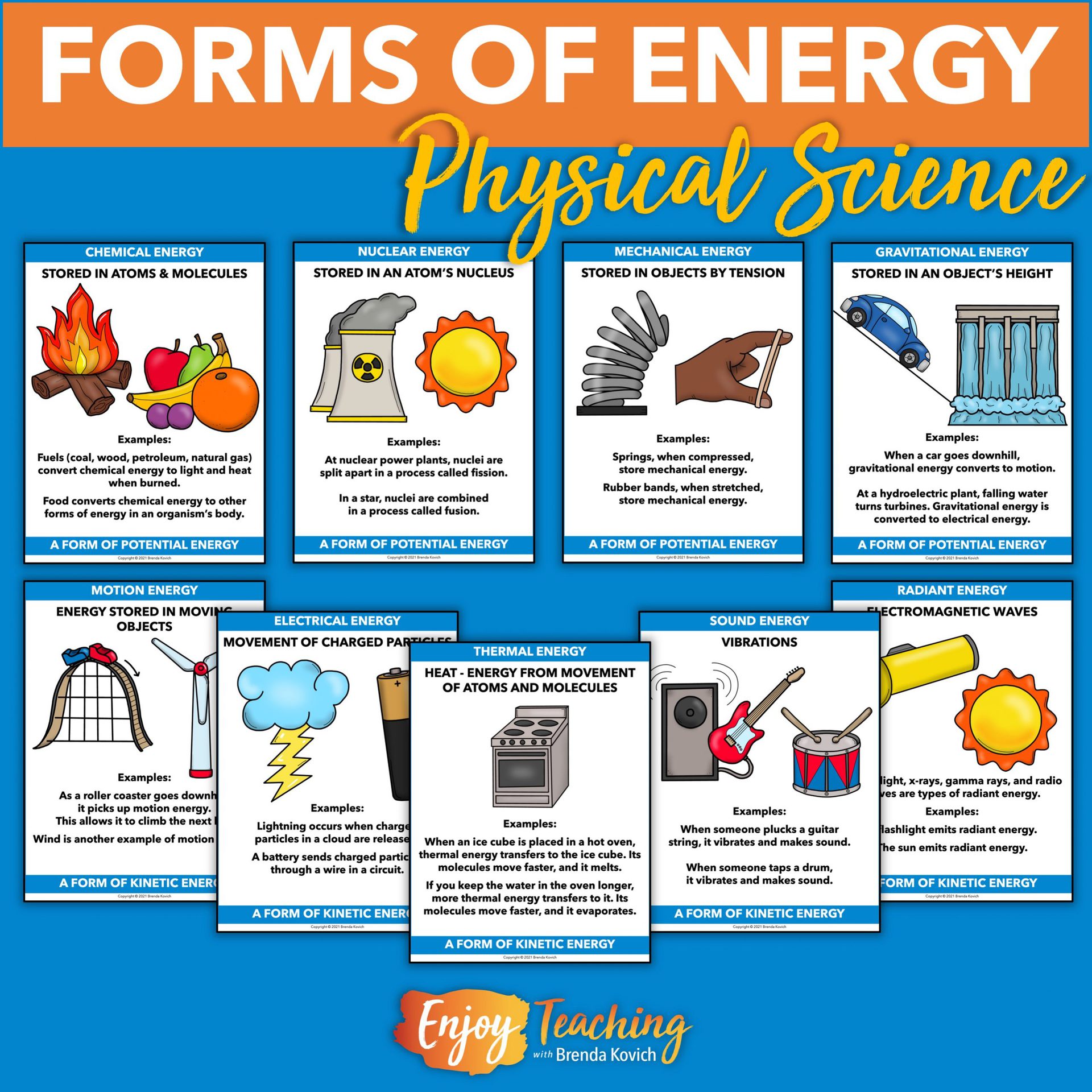
Comments
Post a Comment